
The next element is lithium, with Z = 3 and three electrons in the neutral atom. Otherwise, our configuration would violate the Pauli principle. Written as 1 s 2, where the superscript 2 implies the pairing of spins. The orbital diagram for the helium atom is therefore From the Pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down, so that the electrons are paired. We place one electron in the orbital that is lowest in energy, the 1 s orbital. Unless there is a reason to show the empty higher energy orbitals, these are often omitted in an orbital diagram: Figure 2.1.1), and the electron configuration is written as 1 s 1 and read as “one-s-one.”Ī neutral helium atom, with an atomic number of 2 ( Z = 2), has two electrons. Some authors express the orbital diagram horizontally (removing the implicit energy axis and the colon symbol): Here is a schematic orbital diagram for a hydrogen atom in its ground state: A filled orbital is indicated by ↑↓, in which the electron spins are said to be paired. We use the orbital energy diagram of Figure 2.1.1, recognizing that each orbital can hold two electrons, one with spin up ↑, corresponding to m s = +½, which is arbitrarily written first, and one with spin down ↓, corresponding to m s = −½. First we determine the number of electrons in the atom then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli principle.

We construct the periodic table by following the aufbau principle (from German, meaning “building up”). Web electron configurations are a simple way of writing down the locations of all of the electrons in an atom.\) Web orbital diagram for carbon (c) | carbon electron configuration. Web the shorthand electron configuration for carbon is 2s 2 2p 2. The Total Number Of Electrons In Carbon Is Six. Web carbon is situated in group 14th and has an atomic number of 6. Web to write the orbital diagram for the carbon atom (c) first we need to write the electron configuration for just c. Web for carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. Two In The First Shell, Eight In The Second Shell, Eight In The Third Shell. If you guys have come across our recent article then it would be. This is important when describing an electron configuration in terms of the orbital diagrams. In the co2 lewis structure, there are a total of. Web the expanded notation for carbon is written as follows: Carbon Typically Shares Electrons To Achieve A Complete Valence Shell, Forming Bonds With Multiple Other. Web carbon is the 6th element in the periodic table and its symbol is ‘c’. Web The Valence Electron For Carbon (1S22S22P2) And Hydrogen (1S1) Is 4 And 1, Respectively. Web the carbon (c) atom is kept at the central position and the oxygen (o) atom is on either side of it in the lewis diagram. Web the expanded notation for carbon is written as follows: Carbon (Atomic Number Z=6) In An Unbonded.Īs usual, we will draw two dots together on one side, to represent the.

Source: Check DetailsĬarbon is a member of group 14 and period 2 of the periodic table. The first shell of carbon has 2 electrons and the outer shell or valence shell of carbon has 4. Two in the first shell, eight in the second shell, eight in the third shell. The total number of electrons in carbon is six. Web count the number of lone pairs + the number of atoms that are directly attached to the central atom. Web electron configurations are a simple way of writing down the locations of all of the electrons in an atom. 1.14 1.17 Atomic structure from Web count the number of lone pairs + the number of atoms that are directly attached to the central atom.

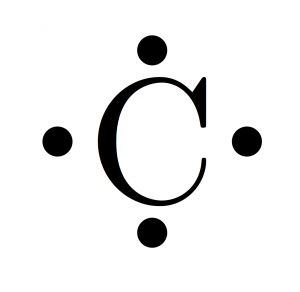
(or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around.


 0 kommentar(er)
0 kommentar(er)
